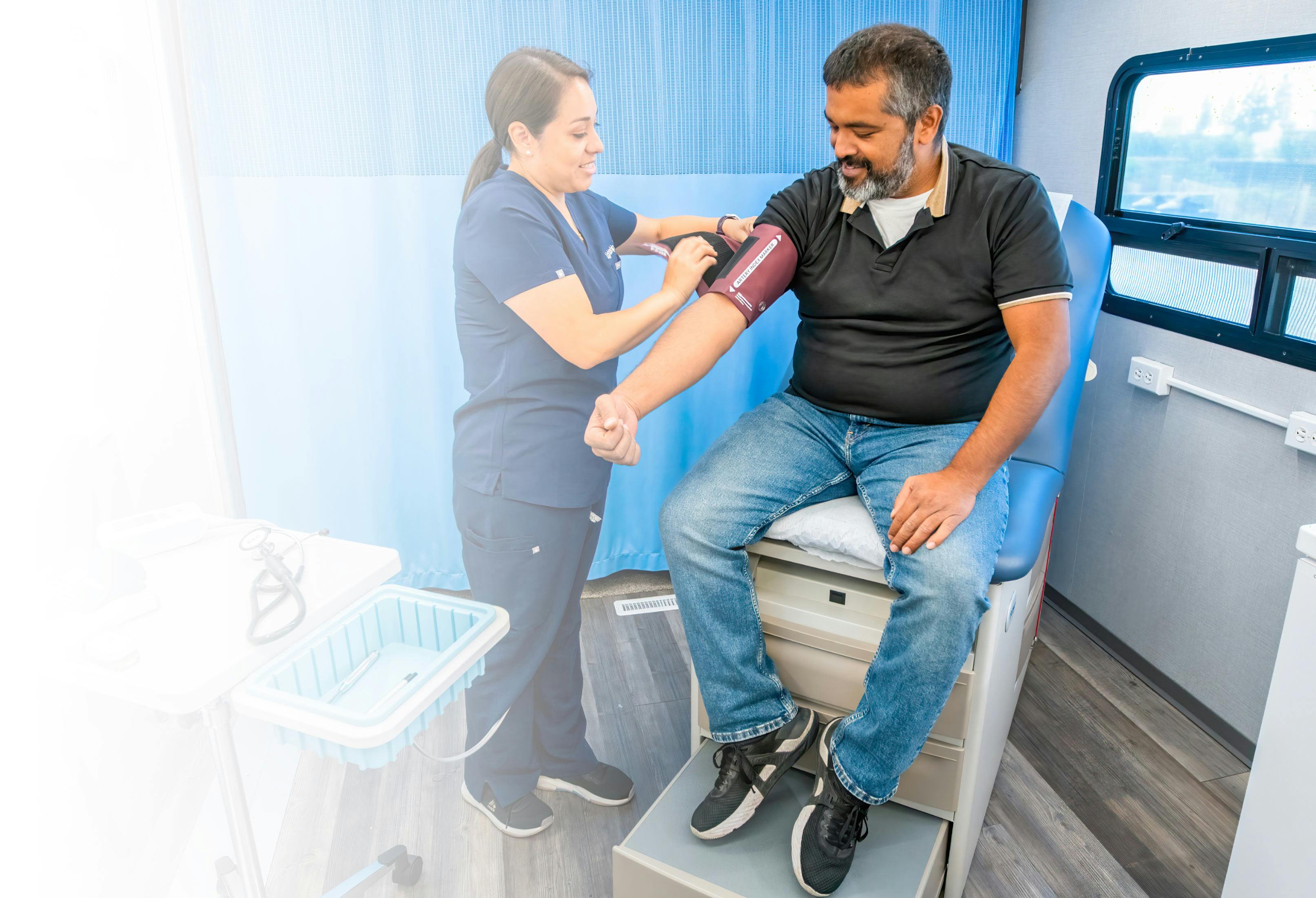
Hybrid Trial Services
Access nationwide research sites to deliver end-to-end hybrid studies. Expand reach, reduce travel burdens, and increase patient participation.

Redefining Trials for Convenience and Quality
Lightship hybrid trials break down participation barriers, providing inclusive opportunities for all patient populations, particularly those not living close to a clinic. By combining traditional site visits with decentralized elements, our approach allows sponsors to deliver
on protocol and participant needs.
Our national network of sites and seamless integration with
remote visits offer flexible participation, validating endpoints while minimizing travel burdens. This simultaneously expands recruitment and delivers reliable, efficient results.
Blending The Best In Study Methods
Hybrid trial services blend traditional and decentralized methods to provide flexibility and efficiency. With a network of 30+ Lightship research sites across the US, experienced investigators, and advanced technology, we deliver high-quality, patient-centric research that leverages the strengths of both approaches.
Lightship accesses 3x more patients with a hybrid approach compared to a traditional brick-and-mortar model, accelerating enrollment.
40% of leads identified and engaged are from diverse backgrounds, ensuring inclusivity and representation.
Using Lightship’s pre-qualified, trained and contracted research sites allows for rapid start-up with average timelines of 6 weeks from site identification to SIV.
With a subset of visits conducted in the home or off-site with investigator oversight, site capacity is freed up for routine care.
Lightship’s Site Network
Lightship’s curated network of 30+ research sites, along with additional partner sites across the U.S., brings clinical trials directly to local communities. Our experienced sites support a wide range of studies with on-site visits and remote oversight. Each site is pre-qualified, trained, and contracted, ensuring rapid activation and seamless trial execution.
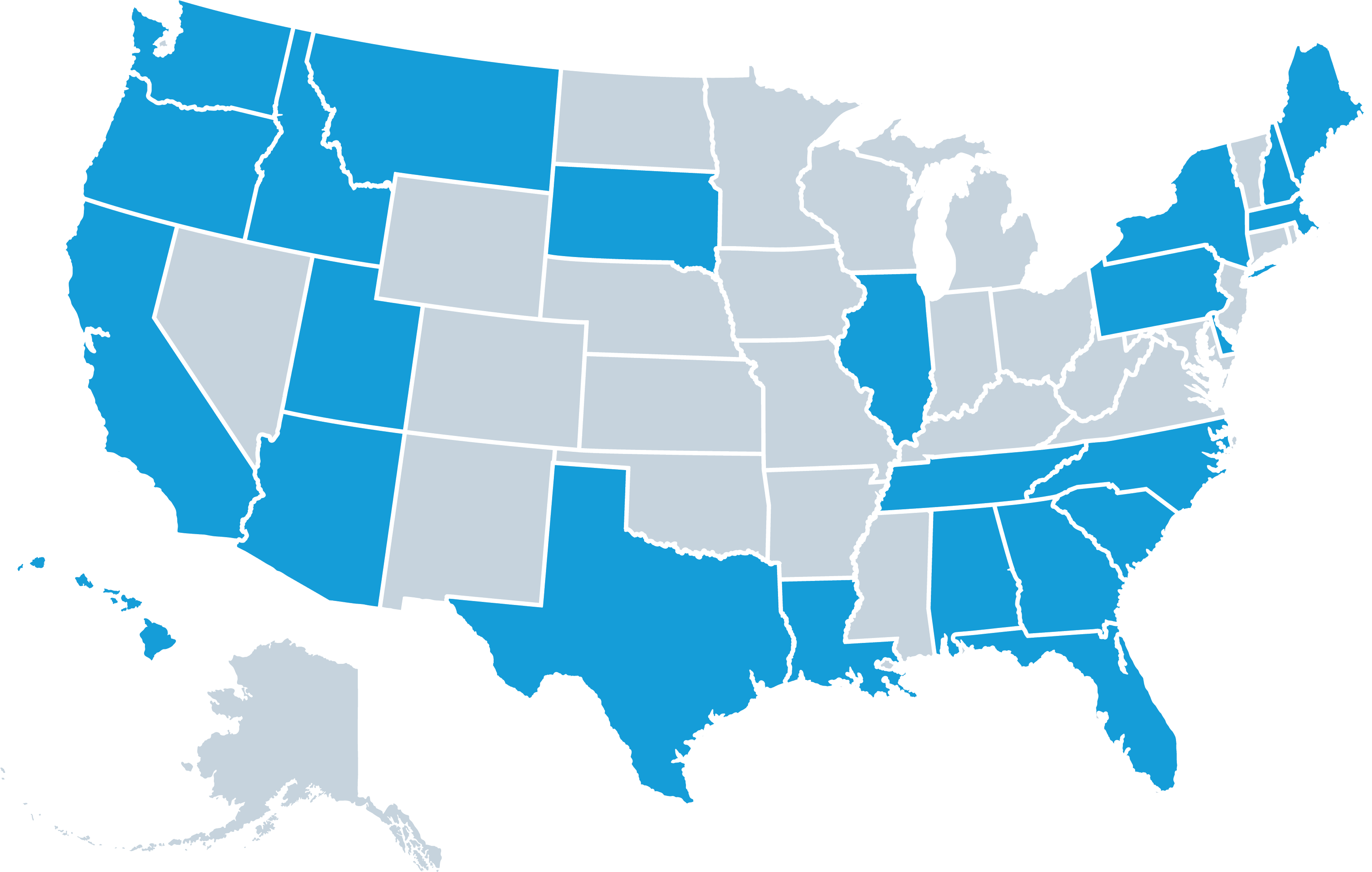
Lightship Site and Investigator Coverage
How It Works
We design hybrid trial services to deliver inclusive, flexible, and clinical trials. By blending traditional and decentralized methods, we ensure patients receive personalized care, maximize participation, and achieve high-quality, real-time results. Here’s how it works.

We tailor each study to incorporate decentralized elements into a traditional brick-and-mortar model, where appropriate. We then select the right sites to deliver on your study goals from our Lightship Site Network, allowing for rapid study start-up. If necessary, we work with existing sponsor-selected sites.

We use targeted outreach in the community, digital channels and site databases to identify potential participants. Our centralized research coordinators and nurses rapidly pre-screen potential participants, referring qualified individuals to the site for enrollment. Our team remains engaged with participants throughout the duration of the study, allowing for a single point of contact.

Lightship oversees selected sites from start-up to close-out, ensuring seamless integration of site-based and remote activities. Our research nurses serve as an extension of the site with remote PI oversight and conduct agreed study visits and assessments in the home. Where needed, our research staff also provides on-site support. This is supported by tools that enable real-time, electronic and remote data transfer to the site EDC.
Case Study
37% Minority Participation and 63% Expanded Reach in Cardiology Trial
Lightship partnered with a top-10 pharmaceutical company to address low minority participation and geographic challenges in a hybrid cardiology study. The trial required recruiting 50 patients across seven sites, delivering injectable medications, and reducing the travel burden for participants.
Lightship implemented a tailored strategy, combining traditional and decentralized sites. Remote PI oversight ensured clinical integrity, while mobile research nurses conducted in-home treatment visits. Through expert study management, Lightship achieved study milestones ahead of schedule, expanded participant reach, and increased trial representation.
As a result, the client:
- Recruited 37% of participants identifying as Black, surpassing the typical 6% in cardiology trials
- Expanded participant reach by 63%, increasing the average distance from study sites
- Achieved seamless monitoring visits with zero concerns reported
- Met recruitment goals on time, ensuring the study stayed on track

Integrated clinical trial services
Lightship offers tailored services designed to meet your unique clinical trial needs. Whether you integrate seamlessly or use them as independent solutions, we help you ensure the best outcome

Outreach & Recruitment
Engaging and enrolling participants in the community
Decentralized Trial Services
Expanding trials beyond traditional sites
Mobile Research Unit
Bringing research directly to communities
Mobile Nursing Services
Ensuring in-home patient supportWhy Lightship?
Lightship combines the best aspects of traditional and decentralized trial methods, tailored to fit each unique situation for maximum results. Our approach ensures:
Reach Any Patient, Anywhere
At Lightship, we connect with patients wherever they are. Blending traditional and decentralized methods, we ensure each trial reaches the right participants.
Let’s discuss how we can create tailored solutions that bring out the best in your trial.