
Decentralized Clinical Trial Services
Our solutions bring research closer to patients – removing geographic barriers, increasing access, and accelerating enrollment.

Breaking Down Barriers
Virtual site models eliminate geographic barriers, ensuring study activities are conducted wherever patients are. From recruitment to real-time data transfer, Lightship prioritizes accessibility, convenience, and results for patients and sponsors.
What DCT was Meant to Be
By combining clinical expertise with innovative technology, Lightship delivers efficient, patient-centric trials that drive better retention, compliance, and high-quality data.
2.5x Faster Enrollment
With no geographic barriers, Lightship’s decentralized studies make participation accessible to over 80% of the population increasing the speed of enrollment.40% Diverse Engagement
40% of engaged leads come from diverse backgrounds, promoting inclusivity and representation.95% Retention
95% average patient retention, with 100% satisfaction for home visits, reflects the quality of our personalized care.How It Works
With deep expertise, proven methodologies, and an unwavering commitment to quality, we conduct decentralized trials that enhance engagement, retention, and data integrity. Our comprehensive service model covers all activities from study design and start-up to conduct and close-out. Here’s how we do it.

We tailor each study by integrating decentralized elements that align with the unique needs of sponsors and patients, ensuring clinical trial endpoints are met while maintaining both convenience and regulatory rigor.

We use targeted outreach in communities, digital channels, partnerships and patient databases to identify potential participants. Our centralized research coordinators and nurses rapidly pre-screen potential participants and organize necessary screening visits at home. Our team remains engaged with participants throughout the duration of the study, allowing for a single point of contact.

Our team of research nurses conduct study visits and necessary protocol assessments at home or a preferred alternative location, with remote PI oversight. These visits may include patient education, training, and IMP preparation or administration. Supported by advanced technology, we ensure seamless electronic data collection and secure transfer for efficient trial management.
Case Study
Rapid Launch of Fully Decentralized Dermatology Study with 100% Patient Satisfaction
Lightship partnered with a top-10 pharmaceutical company to execute a fully decentralized dermatology trial.
This rescue project required a rapid start-up and recruitment of 100 patients for a fully remote study across five US states.
To ensure a seamless experience, the client employed Lightship’s decentralized trial services to manage site ID, qualification, start-up, conduct, and close-out activities. A dedicated remote PI led the trial, supported by a team of NP/PA and RN professionals who conducted assessments and procedures directly in patients’ homes.
Key results:
- Study launched within 6 weeks of protocol receipt
- Recruitment targets were exceeded and ahead of schedule, with an average distance of 420 miles between PI and participants
- 288 home visits conducted by Lightship employed research nurses ensuring continuity of care
- 100% of participants felt comfortable with Lightship study personnel in their home
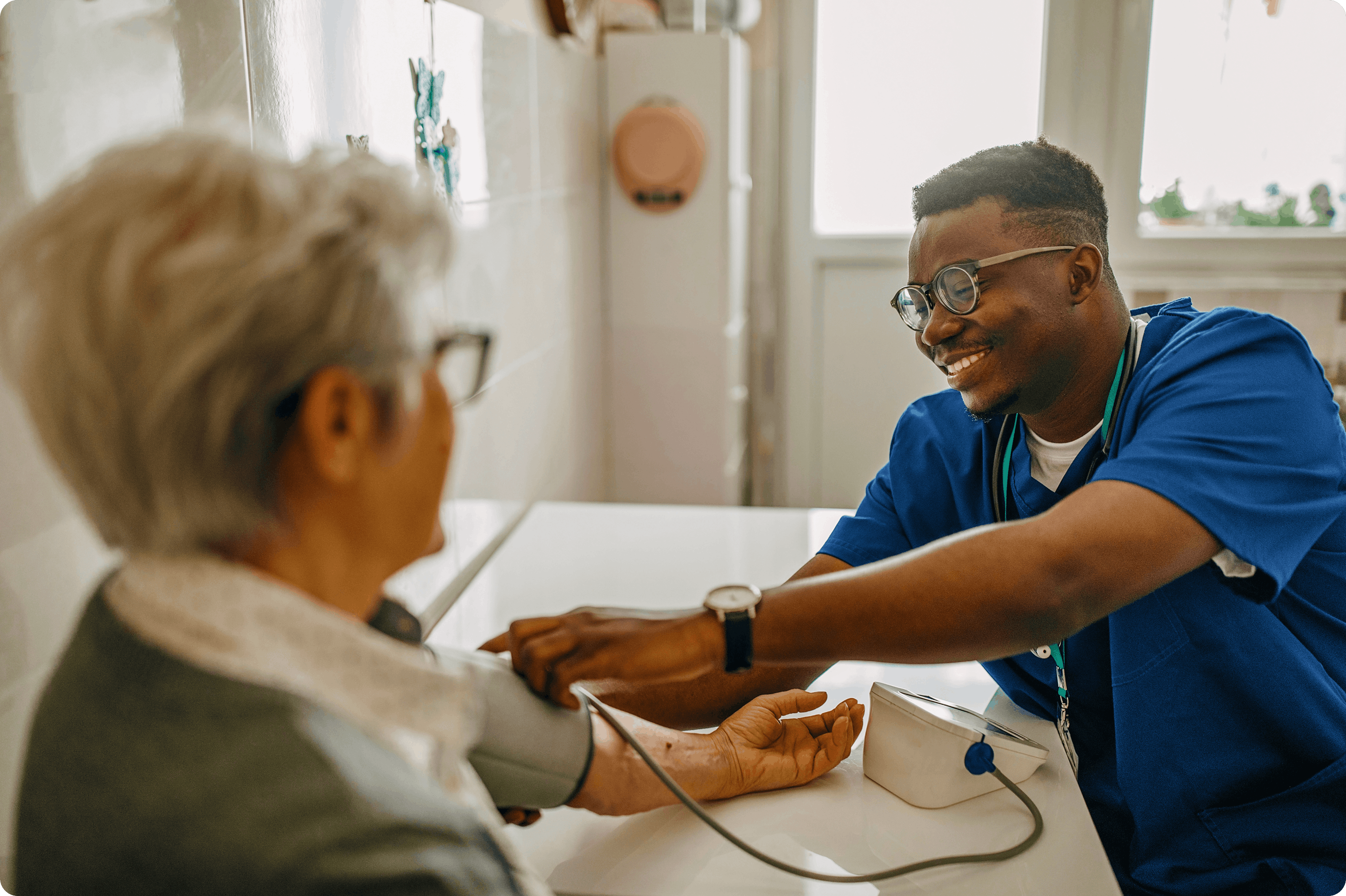
Integrated Clinical Trial Services
Lightship offers modular services tailored to your trial needs - available as standalone solutions or seamlessly integrated into a full-service decentralized model.

Outreach & Recruitment
Engaging and enrolling participants in the community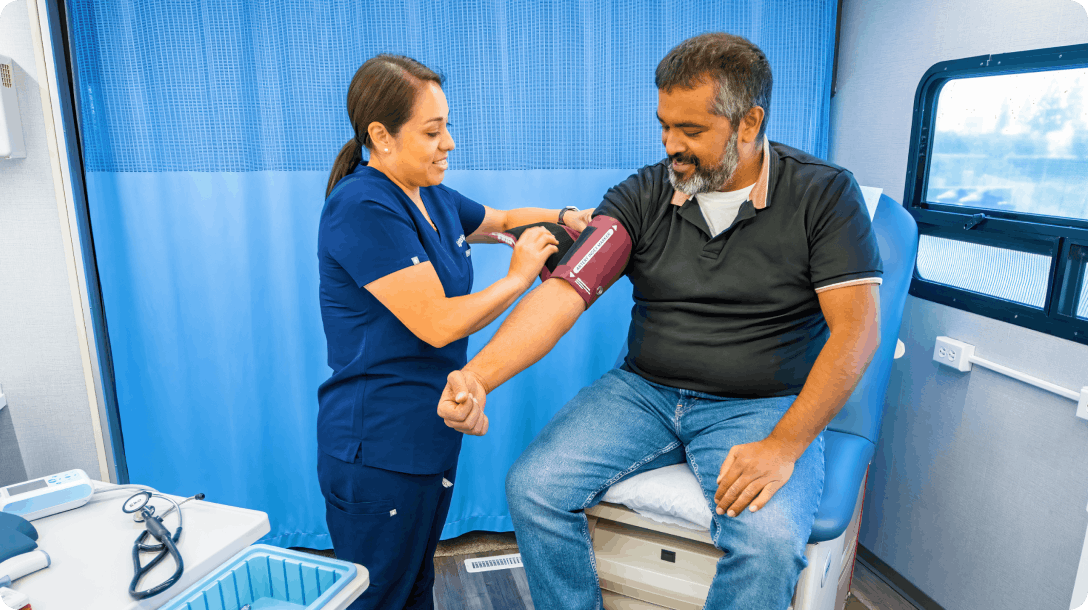
Hybrid Trial Services
Combining site-based and remote participation
Mobile Research Unit
Bringing research directly to communities
Mobile Nursing Services
Ensuring in-home patient supportWhy Lightship?
Lightship combines innovation and expertise to deliver decentralized clinical trials that empower patients and sponsors alike. Our approach ensures:
Your Trial,
Delivered Directly
At Lightship, we redefine trial participation, bringing the study directly to patients.
Let’s explore how we can design a trial that expands access, enhances engagement, and ensures seamless data collection.