
Inclusive Clinical Trial Services
Access, illuminated.
Conduct inclusive decentralized and hybrid clinical trials faster and more effectively with Lightship. A community-based clinical trial solution for accessing and enrolling representative populations at nationwide scale, all while keeping patients at the center from start-up to close-out.
Accelerate inclusive enrollment & delivery
Better access requires not just connectivity, but connection. Lightship's proven model takes you farther than technology alone can reach, traversing geographic and cultural barriers to deliver a faster and more representative trial, underpinned by the highest quality standards.
Access three times the patient reach of traditional brick-and-mortar trials.
Get your trials up and running twice as fast with Lightship's Site Network.
Lightship's hybrid and DCT trials provide over 20% better patient retention than traditional trials.
On average, 40% of the leads we enroll identify as being from racial or ethnically diverse groups.
Our Solutions
Clinically grounded. Culturally competent.
Modern clinical trials require both clinical and cultural competence. With Lightship, you get both, supported by an expert team with decades of hands-on experience delivering proven results and balancing speed with quality. We specialize in serving the unique needs of biopharmaceutical sponsors, academic institutions and CROs across the US and UK.

Outreach & Recruitment
Educate, qualify and enroll representative populations faster with our targeted community-based outreach model.

Decentralized Trials
Deliver trials without geographical boundaries, increasing enrollment and participation amongst traditionally underserved populations. Discover what DCT was meant to be with Lightship.
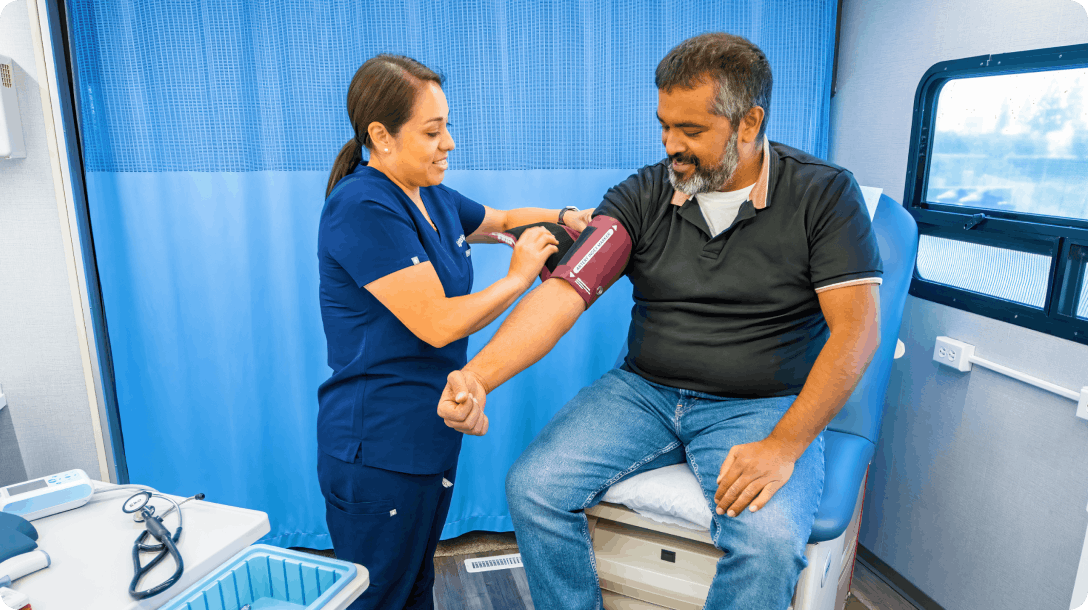
Hybrid Trials
Seamlessly run a hybrid study by bringing together our remote offering and extensive, curated network of experienced investigators and sites built for inclusive studies.

Mobile Research Units
Conduct engagement, outreach, pre-screening and study visits in a clinic setting in the community with a convenient alternative to the home.

Mobile Nurses
Achieve better consistency and quality of care with study visits delivered at home or an alternative location of choice by research nurses who only work with us.
Therapeutic Areas
Our clinical trial experience and expertise spans a number of different therapies, with a focus on phase 2 and 3 trials and observational studies.
Success Stories